top of page
At Emmecell, we are committed to curing blindness. Our mission is to bring innovative regenerative medicine out of the laboratory and into patient care.
We are harnessing the power of our platform to discover and develop new cell-based therapies that have the
potential to transform the lives of patients with serious eye diseases.
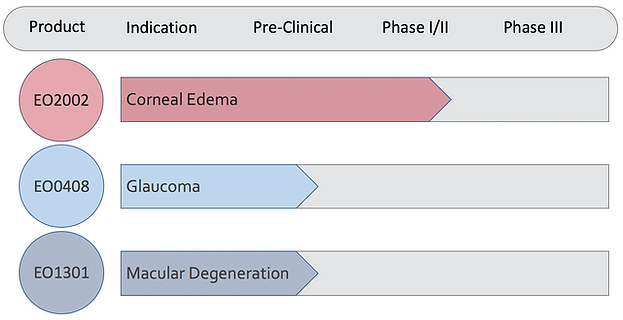
Product Pipeline
Clinical Trials
EO2002 - Magnetic Human Corneal Endothelial Cells for Corneal Edema
Emmecell’s clinical programs are designed to drive key value inflection points as its magnetic cell delivery platform advances toward pivotal development. The company is preparing to launch a pivotal clinical program in corneal disease.
For more information about our clinical trials, contact us at clinicaltrials@emmecell.com.
bottom of page
